cell comparison
next lesson
summary
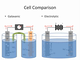
the content provides a comparative analysis of galvanic and electrolytic cells, focusing on their operational differences and the nature of electron flow within each.
- galvanic cells operate spontaneously with a negative delta g, while electrolytic cells require external energy to proceed due to their positive delta g.
- in both cell types, the anode undergoes oxidation and the cathode undergoes reduction, with electrons flowing from the anode to the cathode.
- the major difference lies in the electron flow: it is free in galvanic cells but forced in electrolytic cells, necessitating an external power source.
- the charges on the anodes and cathodes are opposite in the two types of cells, affecting the direction and necessity of electron flow.
chapters
00:00
understanding galvanic cells
00:44
electron flow in cells